Nutraceutical Compounds as Sensitizers for Cancer Treatment in Radiation Therapy
Abstract
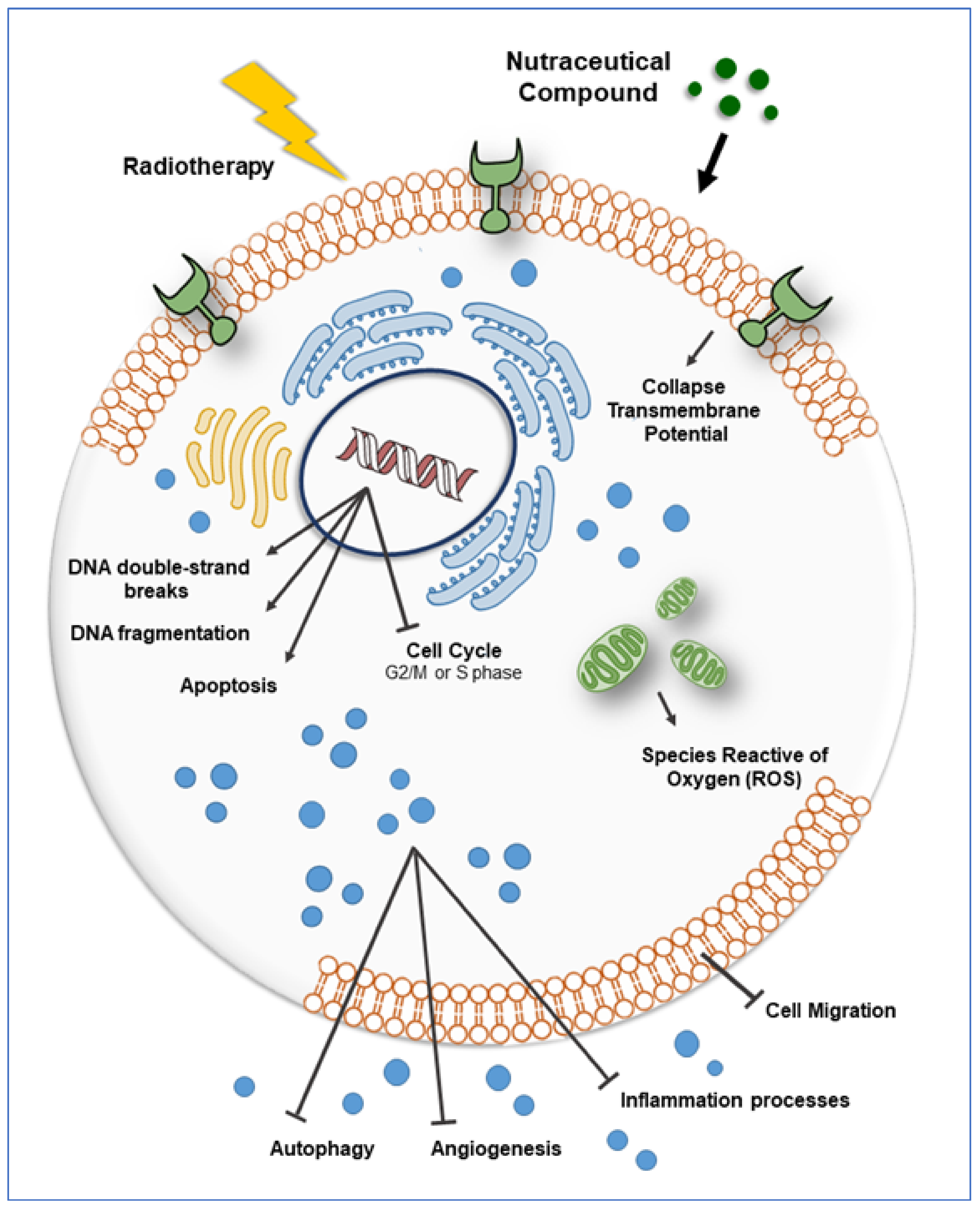
:1. Introduction
2. Curcumin
3. Resveratrol
4. Withaferin A
5. Celastrol
6. Ursolic Acid
7. Zerumbone
8. Caffeic Acid Phenethyl Ester
9. Emodin
10. Flavopiridol
11. Berberine
12. Genistein
13. Selenium
14. Discussion
15. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Forte, G.I.; Minafra, L.; Bravatà, V.; Cammarata, F.P.; Lamia, D.; Pisciotta, P.; Cirrone, G.A.P.; Cuttone, G.; Gilardi, M.C.; Russo, G.; et al. Radiogenomics: The utility in patient selection. Transl. Cancer Res. 2017, 65, 852–S874. [Google Scholar] [CrossRef]
- Bravatà, V.; Cava, C.; Minafra, L.; Cammarata, F.P.; Russo, G.; Gilardi, M.C.; Castiglioni, I.; Forte, G.I. Radiation-Induced Gene Expression Changes in High and Low Grade Breast Cancer Cell Types. Int. J. Mol. Sci. 2018, 19, 1084. [Google Scholar] [CrossRef] [PubMed]
- Baskar, R.; Lee, A.; Yeo, R.; Yeoh, W. Cancer and radiation therapy: Current advances and future directions. Int. J. Med. Sci. 2012, 9, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Grimes, R.D.; Partridge, M. A mechanistic investigation of the oxygen fixation hypothesis and oxygen enhancement ratio. Biomed. Phys. Eng. Express 2015, 1, 045209. [Google Scholar] [CrossRef]
- Rauth, A.M. Pharmacology and toxicology of sensitizers: Mechanism studies. Int. J. Radiat. Oncol. Biol. Phys. 1984, 10, 1293–1300. [Google Scholar] [CrossRef]
- Bonnet, M.; Hong, C.R.; Wong, W.W.; Liew, L.P.; Shome, A.; Wang, J.; Gu, Y.; Stevenson, R.J.; Qi, W.; Anderson, R.F.; et al. Next-Generation Hypoxic Cell Radiosensitizers: Nitroimidazole Alkylsulfonamides. J. Med. Chem. 2018, 61(3), 1241–1254. [Google Scholar] [CrossRef]
- Iliakis, G.; Kurtzman, S. Application of non-hypoxic cell sensitizers in radiobiology and radiotherapy: Rationale and future prospects. Int. J. Radiat. Oncol. Biol. Phys. 1989, 165, 1235–1241. [Google Scholar] [CrossRef]
- Walle, T.; Martinez Monge, R.; Cerwenka, A.; Ajona, D.; Melero, I.; Lecanda, F. Radiation effects on antitumor immune responses: Current perspectives and challenges. Ther. Adv. Med. Oncol. 2018. [Google Scholar] [CrossRef]
- Schölch, S.; Rauber, C.; Tietz, A.; Rahbari, N.N.; Bork, U.; Schmidt, T.; Kahlert, C.; Haberkorn, U.; Tomai, M.A.; Lipson, K.E.; et al. Radiotherapy combined with TLR7/8 activation induces strong immune responses against gastrointestinal tumors. Oncotarget 2015, 6(7), 4663–4676. [Google Scholar] [CrossRef]
- Choi, C.W.; Jeong, M.H.; Park, Y.S.; Son, C.H.; Lee, H.R.; Koh, E.K. Combination Treatment of Stereotactic Body Radiation Therapy and Immature Dendritic Cell Vaccination for Augmentation of Local and Systemic Effects. Cancer Res. Treat. 2019, 51(2), 464–473. [Google Scholar] [CrossRef]
- Prasad, S.; Aggarwal, B.B. Turmeric, the Golden Spice: From Traditional Medicine to Modern Medicine, 2nd ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2011. [Google Scholar]
- Khazaei Koohpar, Z.; Entezari, M.; Movafagh, A.; Hashemi, M. Anticancer Activity of Curcumin on Human Breast Adenocarcinoma: Role of Mcl-1 Gene. Iran. J. Cancer Prev. 2015, 8(3), e2331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shehzad, A.; Lee, J.; Lee, Y.S. Curcumin in various cancers. Biofactors 2013, 39(1), 56–68. [Google Scholar] [CrossRef] [PubMed]
- Chendil, D.; Ranga, R.S.; Meigooni, D.; Sathishkumar, S.; Ahmed, M.M. Curcumin confers radiosensitizing effect in prostate cancer cell line PC-3. Oncogene 2004, 23, 1599–1607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandur, S.K.; Deorukhkar, A.; Pandey, M.K.; Pabón, A.M.; Shentu, S.; Guha, S.; Aggarwal, B.B.; Krishnan, S.; Krishnan, S. Curcumin modulates the radiosensitivity of colorectal cancer cells by suppressing constitutive and inducible NF-kappaB activity. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 534–542. [Google Scholar] [CrossRef]
- Qian, Y.; Ma, J.; Guo, X.; Sun, J.; Yu, Y.; Cao, B.; Zhang, L.; Ding, X.; Huang, J.; Shao, J.F. Curcumin enhances the radiosensitivity of U87 cells by inducing DUSP-2 Up-regulation. Cell Physiol. Biochem. 2015, 35, 1381–1393. [Google Scholar] [CrossRef]
- Minafra, L.; Porcino, N.; Bravatà, V.; Gaglio, D.; Bonanomi, M.; Amore, E.; Cammarata, F.P.; Russo, G.; Militello, C.; Savoca, G.; et al. Radiosensitizing effect of Curcumin-loaded lipid nanoparticles in breast cancer cells. Nat. Scie. Rep. 2019, 9, 11134. [Google Scholar] [CrossRef]
- Khafif, A.; Lev-Ari, S.; Vexler, A.; Barnea, I.; Starr, A.; Karaush, V.; Haif, S.; Ben-Yosef, R. Curcumin: A potential radio-enhancer in head and neck cancer. Laryngoscope 2009, 119, 2019–2026. [Google Scholar] [CrossRef]
- Renaud, S.; De Lorgeril, M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet 1992, 339, 1523–1526. [Google Scholar] [CrossRef]
- Zhang, C.X.; Liu, Y.M.; Chen, K.L.; Chen, G. A comparative study of anti-aging properties and mechanism: Resveratrol and caloric restriction. Oncotarget 2017, 8, 65717–65729. [Google Scholar]
- Ko, J.H.; Sethi, G.; Um, J.Y.; Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Bishayee, A.; Ahn, K.S. The Role of Resveratrol in Cancer Therapy. Int. J. Mol. Sci. 2017, 18, 12. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedicines 2018, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Dudley, J.I.; Das, D.K. Dose-dependency of resveratrol in providing health benefits. Dose Response 2010, 8(4), 478–500. [Google Scholar] [CrossRef] [PubMed]
- Varoni, E.M.; Lo Faro, A.F.; Sharifi-Rad, J.; Iriti, M. Anticancer Molecular Mechanisms of Resveratrol. Front. Nutr. 2016, 3, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, G.E.; Ivanov, V.N.; Hei, T.K. Radiosensitization of melanoma cells through combined inhibition of protein regulators of cell survival. Apoptosis 2008, 13, 790–802. [Google Scholar] [CrossRef]
- Luo, H.; Yang, A.; Schulte, B.A.; Wargovich, M.J.; Wang, G.Y. Resveratrol Induces Premature Senescence in Lung Cancer Cells via ROS-Mediated DNA Damage. PLoS ONE 2013, 8, e60065. [Google Scholar] [CrossRef]
- Wang, L.; Long, L.; Wang, W.; Liang, Z. Resveratrol, a potential radiation sensitizer for glioma stem cells both in vitro and in vivo. J. Pharmacol. Sci. 2015, 129, 216–225. [Google Scholar] [CrossRef] [Green Version]
- Khoei, S.; Shoja, M.; Mostaar, A.; Faeghi, F. Effects of resveratrol and methoxyamine on the radiosensitivity of iododeoxyuridine in U87MG glioblastoma cell line. Exp. Biol. Med. 2016, 241, 1229–1236. [Google Scholar] [CrossRef] [Green Version]
- Baek, S.H.; Ko, J.H.; Lee, H.; Jung, J.; Kong, M.; Lee, J.W.; Lee, J.; Chinnathambi, A.; Zayed, M.E.; Alharbi, S.A.; et al. Resveratrol inhibits STAT3 signaling pathway through the induction of SOCS-1: Role in apoptosis induction and radiosensitization in head and neck tumor cells. Phytomedicine 2016, 23, 566–577. [Google Scholar] [CrossRef]
- Chen, Y.A.; Lien, H.M.; Kao, M.C.; Lo, U.G.; Lin, L.C.; Lin, C.J.; Chang, S.J.; Chen, C.C.; Hsieh, J.T.; Lin, H.; et al. Sensitization of radioresistant prostate cancer cells by resveratrol isolated from arachis hypogaea stems. PLoS ONE 2017, 12, e0169204. [Google Scholar] [CrossRef]
- Tan, Y.; Wei, X.; Zhang, W.; Wang, X.; Wang, K.; Du, B.; Xiao, J. Resveratrol enhances the radiosensitivity of nasopharyngeal carcinoma cells by downregulating E2F1. Oncol. Rep. 2017, 37, 1833–1841. [Google Scholar] [CrossRef]
- da Costa Araldi, I.C.; Bordin, F.P.R.; Cadoná, F.C.; Barbisan, F.; Azzolin, V.F.; Teixeira, C.F.; Baumhardt, T.; da Cruz, I.B.M.; Duarte, M.M.M.F.; Bauermann, L.F. The in vitro radiosensitizer potential of resveratrol on MCF-7 breast cancer cells. Chem. Biol. Interact. 2018, 282, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Tomé-Carneiro, J.; Gonzálvez, M.; Larrosa, M.; Yáñez-Gascón, M.J.; García-Almagro, F.J.; Ruiz-Ros, J.A.; Tomás-Barberán, F.A.; García-Conesa, M.T.; Espín, J.C. Grape resveratrol increases serum adiponectin and downregulates inflammatory genes in peripheral blood mononuclear cells: A triple-blind, placebo-controlled, one-year clinical trial in patients with stable coronary artery disease. Cardiovasc. Drugs Ther. 2013, 27(1), 37–48. [Google Scholar] [CrossRef] [PubMed]
- Kupchan, S.M.; Doskotch, R.W.; Bollinger, P.; McPhail, A.T.; Sim, G.A.; Renauld, J.A.S. The Isolation and Structural Elucidation of a Novel Steroidal Tumor Inhibitor from Acnistus arborescens. J. Am. Chem. Soc. 1965, 87, 5805–5806. [Google Scholar] [CrossRef] [PubMed]
- Shohat, B.; Gitter, S.; Abraham, A.; Lavie, D. Antitumor activity of withaferin A (NSC-101088). Cancer Chemother. Rep. 1967, 51, 271–276. [Google Scholar] [PubMed]
- Lee, I.C.; Choi, B.Y. Withaferin-A--A natural anticancer agent with pleitropic mechanisms of action. Int. J. Mol. Sci. 2016, 17, 290. [Google Scholar] [CrossRef] [PubMed]
- Devi, P.U. Withaferin A: A new radiosensitizer from the Indian medicinal plant Withania somnifera. Int. J. Radiat. Biol. 1996, 69, 193–197. [Google Scholar] [CrossRef]
- Sharada, A.C.; Solomon, F.E.; Devi, P.U.; Udupa, N.; Srinivasan, K.K. Antitumor and radiosensitizing effects of withaferin A on mouse Ehrlich ascites carcinoma in vivo. Acta Oncol. 1996, 35, 95–100. [Google Scholar] [CrossRef]
- Kamath, R.; Rao, B.S.S.; Devi, P.U. Response of a Mouse Fibrosarcoma to Withaferin A and Radiation. Pharm. Pharmacol. Commun. 1999, 5, 287–291. [Google Scholar] [CrossRef]
- Devi, P.U.; Kamath, R.; Rao, B.S. Radiosensitization of a mouse melanoma by withaferin A: In vivo studies. Indian J. Exp. Biol. 2000, 38, 432–437. [Google Scholar]
- Oh, J.H.; Lee, T.J.; Kim, S.H.; Choi, Y.H.; Lee, S.H.; Lee, J.M.; Kim, Y.H.; Park, J.W.; Kwon, T.K. Induction of apoptosis by withaferin A in human leukemia U937 cells through down-regulation of Akt phosphorylation. Apoptosis 2008, 13, 1494–1504. [Google Scholar] [CrossRef]
- Yang, E.S.; Choi, M.J.; Kim, J.H.; Choi, K.S.; Kwon, T.K. Combination of withaferin A and X-ray irradiation enhances apoptosis in U937 cells. Toxicol. Vitr. 2011, 25, 1803–1810. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.S.; Choi, M.J.; Kim, J.H.; Choi, K.S.; Kwon, T.K. Withaferin A enhances radiation-induced apoptosis in Caki cells through induction of reactive oxygen species, Bcl-2 downregulation and Akt inhibition. Chem. Biol. Interact. 2011, 190, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Liu, X.W.; Cai, T.Y.; Cao, J.; Tu, C.X.; Lu, W.; He, Q.J.; Yang, B. Celastrol acts as a potent antimetastatic agent targeting beta1 integrin and inhibiting cell-extracellular matrix adhesion, in part via the p38 mitogen-activated protein kinase pathway. J. Pharmacol. Exp. Ther. 2010, 334, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; DeSano, J.T.; Meng, Y.; Ji, Q.; Ljungman, M.; Lawrence, T.S.; Xu, L. Celastrol potentiates radiotherapy by impairment of DNA damage processing in human prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Bae, S. Enhancement of radiation sensitivity in lung cancer cells by celastrol is mediated by inhibition of Hsp90. Int. J. Mol. Med. 2011, 27, 441–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, H.R.; Seo, W.D.; Pyun, B.J.; Lee, B.W.; Jin, Y.B.; Park, K.H.; Seo, E.K.; Lee, Y.J.; Lee, Y.S. Radiosensitization by celastrol is mediated by modification of antioxidant thiol molecules. Chem. Biol. Interact. 2011, 193, 34–42. [Google Scholar] [CrossRef]
- Jun, H.Y.; Kim, T.H.; Choi, J.W.; Lee, Y.H.; Lee, K.K.; Yoon, K.H. Evaluation of connectivity map-discovered celastrol as a radiosensitizing agent in a murine lung carcinoma model: Feasibility study of diffusion-weighted magnetic resonance imaging. PLoS ONE 2017, 12, e0178204. [Google Scholar] [CrossRef]
- Woźniak, Ł.; Skąpska, S.; Marszałek, K. Ursolic Acid-A Pentacyclic Triterpenoid with a Wide Spectrum of Pharmacological Activities. Molecules 2015, 20, 20614–20641. [Google Scholar] [CrossRef]
- Koh, S.J.; Tak, J.K.; Kim, S.T.; Nam, W.S.; Kim, S.Y.; Park, K.M.; Park, J.W. Sensitization of ionizing radiation-induced apoptosis by ursolic acid. Free Radic. Res. 2012, 46, 339–345. [Google Scholar] [CrossRef]
- Lee, Y.H.; Wang, E.; Kumar, N.; Glickman, R.D. Ursolic acid differentially modulates apoptosis in skin melanoma and retinal pigment epithelial cells exposed to UV–VIS broadband radiation. Apoptosis 2014, 19, 816–828. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, M.; Hu, J.; Lv, X.; Yu, L.; Qian, X.; Liu, B. Enhancement of Radiation Effects by Ursolic Acid in BGC-823 Human Adenocarcinoma Gastric Cancer Cell Line. PLoS ONE 2015, 10, e0133169. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Zhang, Q.; Yu, M.; Qi, X.; Wang, G.; Xiao, L.; Yi, Q.; Jin, W. Ursolic acid sensitizes radioresistant NSCLC cells expressing HIF-1α through reducing endogenous GSH and inhibiting HIF-1α. Oncol. Lett. 2017, 2, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.L.; Liang, J.W.; Hsu, L.C.; Chang, W.L.; Lee, S.S.; Guh, J.H. Zerumbone, a ginger sesquiterpene, induces apoptosis and autophagy in human hormone-refractory prostate cancers through tubulin binding and crosstalk between endoplasmic reticulum stress and mitochondrial insult. Naunyn Schmiedebergs Arch. Pharmacol. 2015, 388, 1223–1236. [Google Scholar] [CrossRef] [PubMed]
- Fusi, F.; Durante, M.; Sgaragli, G.; Khanh, P.N.; Son, N.T.; Huong, T.T.; Cuong, N.M. In vitro vasoactivity of zerumbone from Zingiber zerumbet. Planta Med. 2015, 81, 298–304. [Google Scholar] [CrossRef]
- Yob, N.J.; Joffry, S.M.; Affandi, M.M.; Teh, L.K.; Salleh, M.Z.; Zakaria, Z.A. Zingiber zerumbet (L.) Smith: A review of its ethnomedicinal, chemical, and pharmacological uses. Evid. Based Complement. Altern. Med. 2011, 2011, 543216. [Google Scholar] [CrossRef]
- Sung, B.; Jhurani, S.; Ahn, K.S.; Mastuo, Y.; Yi, T.; Guha, S.; Liu, M.; Aggarwal, B.B. Zerumbone downregulates chemokine receptor CXCR4 expression leading to inhibition of CXCL12-induced invasion of breast and pancreatic tumor cells. Cancer Res. 2008, 68, 8938–8944. [Google Scholar] [CrossRef]
- Murakami, A.; Tanaka, T.; Lee, J.Y.; Surh, Y.J.; Kim, H.W.; Kawabata, K.; Nakamura, Y.; Jiwajinda, S.; Ohigashi, H. Zerumbone, a sesquiterpene in subtropical ginger, suppresses skin tumor initiation and promotion stages in ICR mice. Int. J. Cancer 2004, 110, 481–490. [Google Scholar] [CrossRef]
- Rahman, H.S.; Rasedee, A.; Yeap, S.K.; Othman, H.H.; Chartrand, M.S.; Namvar, F.; Abdul, A.B.; How, C.W. Biomedical properties of a natural dietary plant metabolite, zerumbone, in cancer therapy and chemoprevention trials. Biomed. Res. Int. 2017, 2014, 920742. [Google Scholar] [CrossRef]
- Choi, S.H.; Lee, Y.J.; Seo, W.D.; Lee, H.J.; Nam, J.W.; Lee, Y.J.; Kim, J.; Seo, E.K.; Lee, Y.S. Altered cross-linking of HSP27 by zerumbone as a novel strategy for overcoming HSP27-mediated radioresistance. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 1196–1205. [Google Scholar] [CrossRef]
- Huang, G.C.; Chien, T.Y.; Chen, L.G.; Wang, C.C. Antitumor effects of zerumbone from Zingiber zerumbet in P-388D1 cells in vitro and in vivo. Planta Med. 2005, 71, 219–224. [Google Scholar] [CrossRef]
- Chiang, M.F.; Chen, H.H.; Chi, C.W.; Sze, C.I.; Hsu, M.L.; Shieh, H.R.; Lin, C.P.; Tsai, J.T.; Chen, Y.J. Modulation of Sonic hedgehog signaling and WW domain containing oxidoreductase WOX1 expression enhances radiosensitivity of human glioblastoma cells. Exp. Biol. Med. 2015, 240, 392–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiang, P.K.; Tsai, W.K.; Chen, M.; Lin, W.R.; Chow, Y.C.; Lee, C.C.; Hsu, J.M.; Chen, Y.J. Zerumbone Regulates DNA Repair Responding to Ionizing Radiation and Enhances Radiosensitivity of Human Prostatic Cancer Cells. Int. Cancer Ther. 2018, 17, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Deorukhkar, A.; Ahuja, N.; Mercado, A.L.; Diagaradjane, P.; Raju, U.; Patel, N.; Mohindra, P.; Diep, N.; Guha, S.; Krishnan, S. Zerumbone increases oxidative stress in a thiol-dependent ROS-independent manner to increase DNA damage and sensitize colorectal cancer cells to radiation. Cancer Med. 2015, 4, 278–292. [Google Scholar] [CrossRef] [PubMed]
- Grunberger, D.; Banerjee, R.; Eisinger, K.; Oltz, E.M.; Efros, L.; Caldwell, M.; Estevez, V.; Nakanishi, K. Preferential cytotoxicity on tumor cells by caffeic acid phenethyl ester isolated from propolis. Experienita 1988, 44, 230–232. [Google Scholar] [CrossRef] [PubMed]
- Cotgreave, I.A.; Gerdes, R.G. Recent trend in glutathione biochemistry-glutatione-protein interactions: A molecular link between oxidative stress and cell proliferation? Biochem. Biophys. Res. Commun. 1998, 242, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kudugunti, S.K.; Vad, N.M.; Whiteside, A.J.; Naik, B.U.; Yusuf, M.A.; Srivenugopal, K.S.; Moridani, M.Y. Biochemical mechanism of caffeic acid phenylethyl ester (CAPE) selective toxicity towards melanoma cell lines. Chem. Biol. Interact. 2010, 188, 1–14. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Kao, C.L.; Tsai, P.H.; Tsai, T.H.; Chiou, S.H.; Wu, W.F.; Ku, H.H.; Wong, T.T. Caffeic acid phenethyl ester preferentially enhanced radiosensitizing and increased oxidative stress in medulloblastoma cell line. Childs Nerv. Syst. 2008, 24, 987–994. [Google Scholar] [CrossRef]
- Lin, Y.H.; Chiu, J.H.; Tseng, W.S.; Wong, T.T.; Chiou, S.H.; Yen, S.H. Antiproliferation and radiosensitization of caffeic acid phenethyl ester on human medulloblastoma cells. Cancer Chemother. Pharmacol. 2006, 57, 525–532. [Google Scholar] [CrossRef]
- Chen, Y.J.; Liao, H.F.; Tsai, T.H.; Wang, S.Y.; Shiao, M.S. Caffeic Acid Phenethyl Ester Preferentially Sensitizes CT26 Colorectal Adenocarcinoma to Ionizing Radiation without affecting Bone Marrow Radioresponse. Int. J. Rad. Oncol. Biol. Phys. 2005, 63, 1252–1261. [Google Scholar] [CrossRef]
- Khoram, N.M.; Bigdeli, B.; Nikoofar, A.; Goliaei, B. Caffeic Acid Phenethyl Ester Increases Radiosensitivity of Estrogen Receptor -Positive and -Negative Breast Cancer Cells by Prolonging Radiation-Induced DNA Damage. J. Breast Cancer 2016, 19, 18–25. [Google Scholar] [CrossRef]
- Chen, M.F.; Wu, C.T.; Chen, Y.J.; Keng, P.C.; Chen, W.C. Cell killing and radiosensitization by caffeic acid phenethyl ester (CAPE) in lung cancer cells. J. Rad. Res. 2004, 45, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Zheng, L.Y.; Xiao, W.; Gui, D.; Wang, X.; Wang, N. Emodin ameliorates high glucose induced-podocyte epithelial-mesenchymal transition in vitro and in vivo. Cell Physiol. Biochem. 2015, 35, 1425–1436. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Tian, Y.; Yu, J.; Song, X.; Jia, R.; Cui, Q.; Tong, W.; Zou, Y.; Li, L.; Yin, L.; et al. iTRAQ-based quantitative proteomic analysis reveals multiple effects of Emodin to Haemophilus parasuis. J. Proteom. 2017, 166, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hou, H.X.; Li, D.R.; Cheng, D.H.; Qin, C.M.; Li, W. Enhancement effect of emodin on radiosensitivity of human nasopharyngeal carcinoma transplanted in nude mice. Chin. Pharm. J. 2010, 45, 1331–1334. [Google Scholar]
- Dey, D.; Ray, R.; Hazra, B. Antitubercular and antibacterial activity of quinonoid natural products against multi-drug resistant clinical isolates. Phytother. Res. 2014, 28, 1014–1021. [Google Scholar] [CrossRef]
- Shrimali, D.; Shanmugam, M.K.; Kumar, A.P.; Zhang, J.; Tan, B.K.; Ahn, K.S.; Sethi, G. Targeted abrogation of diverse signal transduction cascades by emodin for the treatment of inflammatory disorders and cancer. Cancer Lett. 2014, 341, 139–149. [Google Scholar] [CrossRef]
- Gu, J.; Cui, C.F.; Yang, L.; Wang, L.; Jiang, X.H. Emodin inhibits colon cancer cell invasion and migration by suppressing epithelial mesenchymal transition via the Wnt/β-catenin pathway. Oncol. Res. 2018, 27, 193–202. [Google Scholar] [CrossRef]
- Hou, H.; Li, D.; Cheng, D.; Li, L.; Liu, Y.; Zhou, Y. Cellular Redox Status Regulates Emodin-Induced Radiosensitization of Nasopharyngeal Carcinoma Cells In Vitro and In Vivo. J. Pharm. 2013, 2013, 218297. [Google Scholar] [CrossRef]
- Luo, J.; Yuan, Y.; Chang, P.; Li, D.; Liu, Z.; Qu, Y. Combination of aloe-emodin with radiation enhances radiation effects and improves differentiation in human cervical cancer cells. Mol. Med. Rep. 2014, 10, 731–736. [Google Scholar] [CrossRef] [Green Version]
- Hwang, S.Y.; Heo, K.; Kim, J.S.; Im, J.W.; Lee, S.M.; Cho, M.; Kang, D.H.; Heo, J.; Lee, J.W.; Choi, C.W.; et al. Emodin attenuates radioresistance induced by hypoxia in HepG2 cells via the enhancement of PARP1 cleavage and inhibition of JMJD2B. Oncol. Rep. 2015, 33, 1691–1698. [Google Scholar] [CrossRef]
- Miller, R.C.; Murley, J.S.; Rademaker, A.W.; Woloschak, G.E.; Li, J.J.; Weichselbaum, R.R.; Grdina, D.J. Very low doses of ionizing radiation and redox associated modifiers affect survivin-associated changes in radiationsensitivity. Free Rad. Biol. Med. 2016, 99, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Mo, Y.; Hou, H.; Li, D.; Liang, Y.; Chen, D.; Zhou, Y. Mitochondrial protein targets of radiosensitisation by 1,8-dihydroxy-3-acetyl-6-methyl-9,10 anthraquinone on nasopharyngeal carcinoma cells. Eur. J. Pharmacol. 2014, 738, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Bible, K.C.; Kaufmann, S.H. Cytotoxic synergy between flavopiridol (NSC 649890, L86-8275) and various antineoplastic agents: The importance of sequence of administration. Cancer Res. 1997, 57, 3375–3380. [Google Scholar] [PubMed]
- Kaur, G.; Stetler-Stevenson, M.; Sebers, S.; Worland, P.; Sedlacek, H.; Myers, C.; Czech, J.; Naik, R.; Sausville, E. Growth inhibition with reversible cell cycle arrest of carcinoma cells by flavone L86-8275. J. Natl. Cancer Inst. 1992, 84, 1736–1740. [Google Scholar] [CrossRef]
- Byrd, J.C.; Shinn, C.; Waselenko, J.K.; Fuchs, E.J.; Lehman, T.A.; Nguyen, P.L.; Flinn, I.W.; Diehl, L.F.; Sausville, E.; Grever, M.R. Flavopiridol induces apoptosis in chronic lymphocytic leukemia cells via activation of caspase-3 without evidence of bcl-2 modulation or dependence on functional p53. Blood 2008, 92, 3804–3816. [Google Scholar] [CrossRef]
- Patel, V.; Senderowicz, A.M.; Pinto, D.; Igishi, T.; Raffeld, M.; Quintanilla-Martinez, L.; Ensley, J.F.; Sausville, E.A.; Gutkind, J.S. Flavopiridol, a novel cyclin-dependent kinase inhibitor, suppresses the growth of head and neck squamous cell carcinomas by inducing apoptosis. J. Clin. Invest. 1998, 102, 1674–1681. [Google Scholar] [CrossRef]
- Chen, R.; Keating, M.J.; Gandhi, V.; Plunkett, W. Transcription inhibition by flavopiridol: Mechanism of chronic lymphocytic leukemia cell death. Blood 2005, 106, 2513–2519. [Google Scholar] [CrossRef]
- Melillo, G.; Sausville, E.A.; Cloud, K.; Lahusen, T.; Varsio, L.; Senderowicz, A.M. Flavopiridol, a protein kinase inhibitor, downregulates hypoxic induction of vascular endothelial growth factor expression in human monocytes. Cancer Res. 1999, 59, 5433–5437. [Google Scholar]
- Motwani, M.; Delohery, T.M.; Schwartz, G.K. Sequential dependent enhancement of caspase activation and apoptosis by flavopiridol on paclitaxel-treated human gastric and breast cancer cells. Clin. Cancer Res. 1999, 5, 1876–1883. [Google Scholar]
- Kim, J.C.; Saha, D.; Cao, Q.; Choy, H. Enhancement of radiation effects by combined docetaxel and flavopiridol treatment in lung cancer cells. Radiother. Oncol. 2004, 71, 213–221. [Google Scholar] [CrossRef]
- Sato, S.; Kajiyama, Y.; Sugano, M.; Iwanuma, Y.; Tsurumaru, M. Flavopiridol as a radio-sensitizer for esophageal cancer cell lines. Dis. Esophagus. 2004, 17, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Shi, J.; Zhang, Z.; Zhang, F.; Ma, R.; Zhao, Y. The radiation-sensitizing effect of flavopiridol in the esophageal cancer cell line Eca109. Oncol. Lett. 2013, 5, 1872–1876. [Google Scholar] [CrossRef] [PubMed]
- Raju, U.; Ariga, H.; Koto, M.; Lu, X.; Pickett, J.; Valdecanas, D.; Mason, K.A.; Milas, L. Improvement of esophageal adenocarcinoma cell and xenograft responses to radiation by targeting cyclin-dependent kinases. Radiother. Oncol. 2006, 80, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.; Omura-Minamisawa, M.; Kang, Y.; Cheng, C.; Inoue, T. Flavopiridol potentiates the cytotoxic effects of radiation in radioresistant tumor cells in which p53 is mutated or Bcl-2 is overexpressed. Int. J. Radiat. Oncol. Biol. Phys. 2008, 71, 1485–1495. [Google Scholar] [CrossRef]
- Lee, J.M.; Bernstein, A. p53 mutations increase resistance to ionizing radiation. Proc. Natl. Acad. Sci. USA 1993, 90, 5742–5746. [Google Scholar] [CrossRef]
- Gilbert, M.; Knox, S. Influence of Bcl-2 overexpression on Na+/K(+)−ATPase pump activity: Correlation with radiation-induced programmed cell death. J. Cell Physiol. 1997, 171, 299–304. [Google Scholar] [CrossRef]
- Khanna, K.K.; Jackson, S.P. DNA double-strand breaks: Signaling, repair and the cancer connection. Nat. Genet. 2001, 27, 247–254. [Google Scholar] [CrossRef]
- Daboussi, F.; Dumay, A.; Delacôte, F.; Lopez, B.S. DNA double-strand break repair signalling: The case of RAD51 post-translational regulation. Cell Signal. 2002, 14, 969–975. [Google Scholar] [CrossRef]
- Saintigny, Y.; Dumay, A.; Lambert, S.; Lopez, B.S. A novel role for the Bcl-2 protein family: Specific suppression of the RAD51 recombination pathway. EMBO J. 2001, 20, 2596–2607. [Google Scholar] [CrossRef]
- Newcomb, E.W.; Ali, M.A.; Schnee, T.; Lan, L.; Lukyanov, Y.; Fowkes, M.; Miller, D.C.; Zagzag, D. Flavopiridol downregulates hypoxia-mediated hypoxia-inducible factor-1a expression in human glioma cells by a proteasome-independent pathway: Implications for in vivo therapy. Neuro-Oncol. 2005, 7, 225–235. [Google Scholar] [CrossRef]
- Newcomb, E.W.; Lymberis, S.C.; Lukyanov, Y.; Shao, Y.; Schnee, T.; Devitt, M.; Rosenstein, B.S.; Zagzag, D.; Formenti, S.C. Radiation sensitivity of GL261 murine glioma model and enhanced radiation response by flavopiridol. Cell Cycle 2006, 5, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Weaver, D.T. Double-strand break repair by Ku70 requires heterodimerization with Ku80 and DNA binding functions. EMBO J. 1997, 16, 6874–6885. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, W.K.; Mortan, R.A. X-ray sensitivity during the cell generation cycle of cultured Chinese hamster cells. Radiat. Res. 1966, 29, 450–474. [Google Scholar] [CrossRef] [PubMed]
- Sedlacek, H.H. Mechanisms of action of flavopiridol. Crit Rev. Oncol. Hematol. 2001, 38, 139–170. [Google Scholar] [CrossRef]
- Weinberg, R.A. The retinoblastoma protein and cell cycle control. Cell 1995, 81, 323–330. [Google Scholar] [CrossRef] [Green Version]
- Peng, J.; Zhu, Y.; Milton, J.T.; Price, D.H. Identification of multiple cyclin subunits of human P-TEFb. Genes Dev. 1998, 12, 755–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, T.; Peng, J.; Lee, G.; Price, D.H.; Flores, O. Cyclin K functions as a CDK9 regulatory subunit and participates in RNA polymerase II transcription. J. Biol. Chem. 1999, 274, 34527–34530. [Google Scholar] [CrossRef]
- Raju, U.; Nakata, E.; Mason, K.A.; Ang, K.K.; Milas, L. Flavopiridol, a cyclin dependent kinase inhibitor, enhances radiosensitivity of ovarian carcinoma cells. Cancer Res. 2003, 63, 3263–3326. [Google Scholar]
- Kim, S.; Wu, H.G.; Shin, J.H.; Park, H.J.; Kim, I.A.; Kim, I.H. Enhancement of radiation effects by flavopiridol in uterine cervix cancer cells. Cancer Res. Treat. 2005, 37, 191–195. [Google Scholar] [CrossRef]
- Camphausen, K.; Brady, K.J.; Burgan, W.E.; Cerra, M.A.; Russell, J.S.; Bull, E.E.; Tofilon, P.J. Flavopiridol enhances human tumor cell radiosensitivity and prolongs expression of gammaH2AX foci. Mol. Cancer Ther. 2004, 3, 409–416. [Google Scholar]
- McAleer, M.F.; Duffy, K.T.; Davidson, W.R.; Kari, G.; Dicker, A.P.; Rodeck, U.; Wickstrom, E. Antisense inhibition of cyclin D1 expression is equivalent to flavopiridol for radiosensitization of zebrafish embryos. Int. J. Radiat. Oncol. Biol. Phys. 2006, 66, 546–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Li, X.; Zou, D.; Liu, W.; Yang, J.; Zhu, N.; Huo, L.; Wang, M.; Hong, J.; Wu, P.; et al. Treatment of type 2 diabetes and dyslipidemia with the natural plant alkaloid berberine. J. Clin. Endocrinol. Metab. 2008, 93, 2559–2565. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Xing, H.; Ye, J. Efficacy of berberine in patients with type 2 diabetes mellitus. Metabolism 2008, 57, 712–717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, W.; Wei, J.; Abidi, P.; Lin, M.; Inaba, S.; Li, C.; Wang, Y.; Wang, Z.; Si, S.; Pan, H. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat. Med. 2004, 10, 1344–1351. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.M.; Liu, A.A.; Li, T.K.; Wu, H.Y.; Desai, S.D.; Mao, Y.; Rubin, E.H.; LaVoie, E.J.; Makhey, D.; Liu, L.F. Selective cytotoxicity of topoisomerase-directed protoberberines against glioblastoma cells. Biochem. Pharmacol. 1998, 56, 1157–1166. [Google Scholar] [CrossRef]
- Lin, C.C.; Yang, J.S.; Chen, J.T.; Fan, S.; Yu, F.S.; Yang, J.L.; Lu, C.C.; Kao, M.C.; Huang, A.C.; Lu, H.F. Berberine induces apoptosis in human HSC-3 oral cancer cells via simultaneous activation of the death receptor-mediated and mitochondrial pathway. Anticancer Res. 2007, 27, 3371–3378. [Google Scholar]
- Hwang, J.M.; Kuo, H.C.; Tseng, T.H.; Liu, J.Y.; Chu, C.Y. Berberine induces apoptosis through a mitochondria/caspases pathway in human hepatoma cells. Arch. Toxicol. 2006, 80, 62–73. [Google Scholar] [CrossRef]
- Lin, J.P.; Yang, J.S.; Lee, J.H.; Hsieh, W.T.; Chung, J.G. Berberine induces cell cycle arrest and apoptosis in human gastric carcinoma SNU-5 cell line. World J. Gastroenterol. 2006, 12, 21–28. [Google Scholar] [CrossRef]
- Mantena, S.K.; Sharma, S.D.; Katiyar, S.K. Berberine, a natural product, induces G1-phase cell cycle arrest and caspase-3-dependent apoptosis in human prostate carcinoma cells. Mol. Cancer Ther. 2006, 5, 296–308. [Google Scholar] [CrossRef]
- Lin, C.C.; Lin, S.Y.; Chung, J.G.; Lin, J.P.; Chen, G.W.; Kao, S.T. Down-regulation of cyclin B1 and up-regulation of Wee1 by berberine promotes entry of leukemia cells into the G2/M-phase of the cell cycle. Anticancer Res. 2006, 26, 1097–1104. [Google Scholar]
- Liu, Z.; Liu, Q.; Xu, B.; Wu, J.; Guo, C.; Zhu, F.; Yang, Q.; Gao, G.; Gong, Y.; Shao, C. Berberine induces p53-dependent cell cycle arrest and apoptosis of human osteosarcoma cells by inflicting DNA damage. Mutat. Res. 2009, 662, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Peng, P.L.; Kuo, W.H.; Tseng, H.C.; Chou, F.P. Synergistic tumor-killing effect of radiation and berberine combined treatment in lung cancer: The contribution of autophagic cell death. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Jiang, H.; Liu, Z.; Wang, Y.; Zhao, M.; Hao, C.; Feng, S.; Guo, H.; Xu, B.; Yang, Q.; et al. Berberine radiosensitizes human esophageal cancer cells by downregulating homologous recombination repair protein RAD51. PLoS ONE 2011, 6, e23427. [Google Scholar] [CrossRef] [PubMed]
- Moeller, B.J.; Dewhirst, M.W. HIF-1 and tumour radiosensitivity. Br. J. Cancer 2006, 95, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Hypoxia-inducible factors: Mediators of cancer progression and targets for cancer therapy. Trends Pharmacol. Sci. 2012, 33, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yang, B.; Cai, J.; Zhang, C.; Zhang, Q.; Xu, L.; Qin, Q.; Zhu, H.; Ma, J.; Tao, G.; et al. Berberine enhances radiosensitivity of esophageal squamous cancer by targeting HIF-1α in vitro and in vivo. Cancer Biol. Ther. 2013, 14, 1068–1073. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, C.; Yang, X.; Yang, B.; Wang, J.; Kang, Y.; Wang, Z.; Li, D.; Huang, G.; Ma, Z.; et al. Berberine inhibits the expression of hypoxia induction factor-1alpha and increases the radiosensitivity of prostate cancer. Diagn. Pathol. 2014, 9, 98. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, X.; Zhang, Q.; Yang, B.; Xu, L.; Qin, Q.; Zhu, H.; Liu, J.; Cai, J.; Tao, G.; et al. Berberine radiosensitizes human nasopharyngeal carcinoma by suppressing hypoxia-inducible factor-1α expression. Acta Otolaryngol. 2014, 134, 185–192. [Google Scholar] [CrossRef]
- Rosser, C.J.; Tanaka, M.; Pisters, L.L.; Tanaka, N.; Levy, L.B.; Hoover, D.C.; Grossman, H.B.; McDonnell, T.J.; Kuban, D.A.; Meyn, R.E. Adenoviral-mediated PTEN transgene expression sensitizes Bcl-2-expressing prostate cancer cells to radiation. Cancer Gene Ther. 2004, 11, 273–279. [Google Scholar] [CrossRef] [Green Version]
- Szotowski, B.; Antoniak, S.; Goldin-Lang, P.; Tran, Q.V.; Pels, K.; Rosenthal, P.; Bogdanov, V.Y.; Borchert, H.H.; Schultheiss, H.P.; Rauch, U. Antioxidative treatment inhibits the release of thrombogenic tissue factor from irradiation- and cytokine-induced endothelial cells. Cardiovasc. Res. 2007, 73, 806–812. [Google Scholar] [CrossRef]
- Bohnke, A.; Westphal, F.; Schmidt, A.; El-Awady, R.A.; Dahm-Daphi, J. Role of p53 mutations, protein function and DNA damage for the radiosensitivity of human tumor cells. Int. J. Radiat. Biol. 2004, 80, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, F. Reactive oxygen species (ROS), troublemakers between nuclear factor-kappaB (NF-kappaB) and c-Jun NH(2)−terminal kinase (JNK). Cancer Res. 2004, 64, 1902–1905. [Google Scholar] [CrossRef] [PubMed]
- Hur, J.M.; Kim, D. Berberine inhibited radioresistant effects and enhanced antitumor effects in the irradiated-human prostate cancer cells. Toxicol. Res. 2010, 26, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.Y.; Jang, J.Y.; Jeon, Y.K.; Chung, D.H.; Kim, Y.G.; Kim, C.W. Syntenin increases the invasiveness of small cell lung cancer cells by activating p38, AKT, focal adhesion kinase and SP1. Exp. Mol. Med. 2014, 46, e90. [Google Scholar] [CrossRef]
- Kong, L.M.; Liao, C.G.; Zhang, Y.; Xu, J.; Li, Y.; Huang, W.; Zhang, Y.; Bian, H.; Chen, Z.N. A regulatory loop involving miR-22, Sp1, and c-Myc modulates CD147 expression in breast cancer invasion and metastasis. Cancer Res. 2014, 74, 3764–3778. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Ye, W.; Wu, J.; Liu, L.; Yang, L.; Gao, L.; Chen, B.; Zhang, F.; Yang, H.; Li, Y. Sp1-CD147 positive feedback loop promotes the invasion ability of ovarian cancer. Oncol. Rep. 2015, 34, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Kang, M.; Qin, Y.T.; Wei, Z.X.; Xiao, J.J.; Wang, R.S. Sp1 is over-expressed in nasopharyngeal cancer and is a poor prognostic indicator for patients receiving radiotherapy. Int. J. Clin. Exp. Pathol. 2015, 8, 6936–6943. [Google Scholar]
- Wang, J.; Kang, M.; Wen, Q.; Qin, Y.T.; Wei, Z.X.; Xiao, J.J.; Wang, R.S. Berberine sensitizes nasopharyngeal carcinoma cells to radiation through inhibition of Sp1 and EMT. Oncol. Rep. 2017, 37, 2425–2432. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Q.; Yang, Q. Radiosensitization effects of berberine on human breast cancer cells. Int. J. Mol. Med. 2012, 30, 1166–1172. [Google Scholar] [CrossRef]
- Uifălean, A.; Schneider, S.; Ionescu, C.; Lalk, M.; Iuga, C.A. Soy Isoflavones and Breast Cancer Cell Lines: Molecular Mechanisms and Future Perspectives. Molecules 2015, 21, E13. [Google Scholar] [CrossRef]
- Spagnuolo, C.; Russo, G.L.; Orhan, I.E.; Habtemariam, S.; Daglia, M.; Sureda, A.; Nabavi, S.F.; Devi, K.P.; Loizzo, M.R.; Tundis, R.; et al. Genistein and Cancer: Current Status, Challenges, and Future Directions. Adv. Nutr. 2015, 6, 408–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banerjee, S.; Li, Y.; Wang, Z.; Sarkar, F.H. Multi-targeted therapy of cancer by genistein. Cancer Lett. 2008, 269, 226–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y. Mechanisms of caspase activation and inhibition during apoptosis. Mol. Cell 2002, 9, 459–470. [Google Scholar] [CrossRef]
- Altieri, D.C. Validating survivin as a cancer therapeutic target. Nat. Rev. Cancer 2003, 3, 46–54. [Google Scholar] [CrossRef]
- Bao, R.; Connolly, D.C.; Murphy, M.; Green, J.; Weinstein, J.K.; Pisarcik, D.A.; Hamilton, T.C. Activation of cancer-specific gene expression by the survivin promoter. J. Natl. Cancer Inst. 2002, 94, 522–528. [Google Scholar] [CrossRef]
- Rödel, F.; Hoffmann, J.; Distel, L.; Herrmann, M.; Noisternig, T.; Papadopoulos, T.; Sauer, R.; Rödel, C. Survivin as a radioresistance factor, and prognostic and therapeutic target for radiotherapy in rectal cancer. Cancer Res. 2005, 65, 4881–4887. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, J.Y.; Pan, J.S.; Han, S.P.; Yin, X.X.; Wang, B.; Hu, G. Combined treatment of ionizing radiation with genistein on cervical cancer HeLa cells. J. Pharmacol. Sci. 2006, 102, 129–135. [Google Scholar] [CrossRef]
- Wang, S.Y.; Yang, K.W.; Hsu, Y.T.; Chang, C.L.; Yang, Y.C. The differential inhibitory effects of genistein on the growth of cervical cancer cells in vitro. Neoplasma 2001, 48, 227–233. [Google Scholar]
- Yashar, C.M.; Spanos, W.J.; Taylor, D.D.; Gercel-Taylor, C. Potentiation of the radiation effect with genistein in cervical cancer cells. Gynecol. Oncol. 2005, 99, 199–205. [Google Scholar] [CrossRef]
- Shin, J.I.; Shim, J.H.; Kim, K.H.; Choi, H.S.; Kim, J.W.; Lee, H.G.; Kim, B.Y.; Park, S.N.; Park, O.J.; Yoon, D.Y. Sensitization of the apoptotic effect of gamma-irradiation in genistein-pretreated CaSki cervical cancer cells. J. Microbiol. Biotechnol. 2008, 18, 523–531. [Google Scholar]
- Liu, X.; Sun, C.; Jin, X.; Li, P.; Ye, F.; Zhao, T.; Gong, L.; Li, Q. Genistein enhances the radiosensitivity of breast cancer cells via G₂/M cell cycle arrest and apoptosis. Molecules 2013, 18, 13200–13217. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.C. Glutathione synthesis. Biochim. Biophys. Acta. 2013, 1830, 3143–3153. [Google Scholar] [CrossRef] [PubMed]
- Majid, S.; Dar, A.A.; Shahryari, V.; Hirata, H.; Ahmad, A.; Saini, S.; Tanaka, Y.; Dahiya, A.V.; Dahiya, R. Genistein reverses hypermethylation and induces active histone modifications in tumor suppressor gene B-cell translocation gene 3 in prostate cancer. Cancer 2010, 116, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Bai, Q.; Zou, L.Y.; Zhang, Q.Y.; Zhou, Y.; Chang, H.; Yi, L.; Zhu, J.D.; Mi, M.T. Genistein Inhibits DNA Methylation and Increases Expression of Tumor Suppressor Genes in Human Breast Cancer Cells. Gen. Chrom. Cancer 2014, 53, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Baird, L.; Llères, D.; Swift, S.; Dinkova-Kostova, A.T. Regulatory flexibility in the Nrf2-mediated stress response is conferred by conformational cycling of the Keap1-Nrf2 protein complex. Proc. Natl. Acad. Sci. USA 2013, 110, 15259–15264. [Google Scholar] [CrossRef] [Green Version]
- Cho, H.Y.; Reddy, S.P.; Debiase, A.; Yamamoto, M.; Kleeberger, S.R. Gene expression profiling of NRF2-mediated protection against oxidative injury. Free Radic. Biol. Med. 2005, 38, 325–343. [Google Scholar] [CrossRef]
- Liu, X.; Sun, C.; Liu, B.; Jin, X.; Li, P.; Zheng, X.; Zhao, T.; Li, F.; Li, Q. Genistein mediates the selective radiosensitizing effect in NSCLC A549 cells via inhibiting methylation of the keap1 gene promoter region. Oncotarget 2016, 7, 27267–27279. [Google Scholar] [CrossRef]
- You, S.; Li, R.; Parco, D.; Xie, M.; Sica, G.L.; Cao, Y.; Xiao, Z.Q.; Deng, X. Disruption of STAT3 by niclosamide reverses radioresistance of human lung cancer. Mol. Cancer Ther. 2014, 3, 606–616. [Google Scholar] [CrossRef]
- Pattingre, S.; Tassa, A.; Qu, X.; Garuti, R.; Liang, X.H.; Mizushima, N.; Packer, M.; Schneider, M.D.; Levine, B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell 2005, 122, 927–939. [Google Scholar] [CrossRef]
- Du, Y.; Ji, X. Bcl-2 down-regulation by small interfering RNA induces Beclin1-dependent autophagy in human SGC-7901 cells. Cell Biol. Internat. 2014, 38, 1155–1162. [Google Scholar] [CrossRef]
- Zhang, Z.; Jin, F.; Lian, X.; Li, M.; Wang, G.; Lan, B.; He, H.; Liu, G.D.; Wu, Y.; Sun, G.; et al. Genistein promotes ionizing radiation-induced cell death by reducing cytoplasmic Bcl-xL levels in non-small cell lung cancer. Sci. Rep. 2018, 8, 328. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M.; Lipinski, B.; Błażejak, S. Application of Sodium Selenite in the Prevention and Treatment of Cancers. Cells 2017, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M.; Lipinski, B. Pathophysiological significance of protein hydrophobic interactions: An emerging hypothesis. Med. Hypotheses 2018, 110, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Schueller, P.; Puettmann, S.; Micke, O.; Volker, S.; Schaefer, U.; Willich, N. Selenium Influences the Radiation Sensitivity of C6 Rat Glioma Cells. Anticancer Res. 2004, 24, 2913–2918. [Google Scholar] [PubMed]
- Chen, F.; Hong Zhang, X.; Dan Hu, X.; Dang Liu, P.; Qian Zhang, H. The effects of combined selenium nanoparticles and radiation therapy on breast cancer cells in vitro. Artif. Cells Nanomed Biotechnol. 2018, 46(5), 937–948. [Google Scholar] [CrossRef]
- Liu, T.; Shi, C.; Duan, L.; Zhang, Z.; Luo, L.; Goel, S.; Cai, W.; Chen, T. A highly hemocompatible erythrocyte membrane-coated ultrasmall selenium nanosystem for simultaneous cancer radiosensitization and precise antiangiogenesis. J. Mater. Chem. B 2018, 6(29), 4756–4764. [Google Scholar] [CrossRef]
- Holsti, L.R. Development of clinical radiotherapy since 1896. Acta Oncol. 1995, 34, 995–1003. [Google Scholar] [CrossRef]
- Alberti, C. Organ-confined prostate carcinoma radiation brachytherapy compared with external either photon- or hadron-beam radiation therapy. Just a short up-to-date. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 769–774. [Google Scholar]
- Cirrone, G.A.P.; Margarone, D.; Maggiore, M.; Anzalone, A.; Borghesi, M.; Bijan Jia, S.; Bulanov, S.S.; Bulanov, S.; Carpinelli, M.; Cavallaro, S.; et al. ELIMED: A new hadron therapy concept based on laser driven ion beams. Laser Acceleration of Electrons, Protons, and Ions II and Medical Applications of Laser-Generated Beams of Particles II and Harnessing Relativistic Plasma Waves III. Proc. SPIE Int. Soc. Opt. Eng. 2013, 8779. [Google Scholar] [CrossRef]
- Islam, M.T. Radiation interactions with biological systems. Int. J. Radiat Biol. 2017, 93, 487–493. [Google Scholar] [CrossRef]
- Das, L.; Bhaumik, E.; Raychaudhuri, U.; Chakraborty, R. Role of nutraceuticals in human health. J. Food Sci. Technol. 2012, 49, 73–183. [Google Scholar] [CrossRef] [PubMed]
- Reed, D.; Raina, K.; Agarwal, R. Nutraceuticals in prostate cancer therapeutic strategies and their neo-adjuvant use in diverse populations. NPJ Precis. Oncol. 2018, 2, 15. [Google Scholar] [CrossRef] [PubMed]
- Malfa, G.A.; Tomasello, B.; Acquaviva, R.; Genovese, C.; La Mantia, A.; Cammarata, F.P.; Ragusa, M.; Renis, M.; Di Giacomo, C. Betula etnensis Raf. (Betulaceae) Extract Induced HO-1 Expression and Ferroptosis Cell Death in Human Colon Cancer Cells. Int. J. Mol. Sci. 2019, 3, 2723. [Google Scholar] [CrossRef] [PubMed]

| Nutraceuticals | Structure and Molecular Formula | Tumor Targets | Type of Treatment | Bibliography |
|---|---|---|---|---|
| Curcumin |  C21H20O6 | Breast cancer, Colonrectal Cancer, Glioblastoma Multiforme, Head and Neck squamous Cancer, Prostate Cancer. | X-rays | [14,15,16,17,18] |
| Resveratrol | 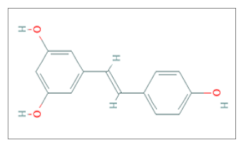 C14H12O3 | Breast Cancer, Glioblastoma, Head and Neck squamous Cancer, Melanoma, Nasopharyngeal Carcinoma, Non-Small Cell Lung Cancer, Prostate Cancer. | γ-rays X-rays | [25,26,27,28,29,30,31,32] |
| Withaferin A |  C28H38O6 | Breast Cancer, Cervical Cancer, Ehrlich Ascites Carcinoma, Fibrosarcoma, Histiocytic Human Lymphoma, Liver Cancer, Melanoma, Renal Carcinoma. | γ-rays X-rays | [37,38,39,40,41,42,43] |
| Celastrol | 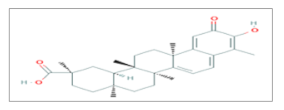 C29H38O4 | Lung Cancer, Prostate Cancer. | γ-rays X-rays | [45,47,48] |
| Ursolic Acid | 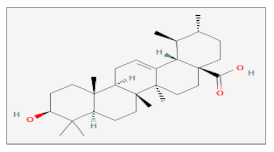 C30H48O3 | Colon Carcinoma, Gastric Adenocarcinoma, Melanoma, Non-Small Cell Lung Cancer, Prostate Cancer. | γ-rays X-rays | [50,51,52,53] |
| Zerumbone | 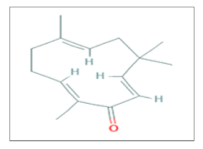 C15H22O | Colonrectal Cancer, Glioblastoma, Lung adenocarcinoma, Non-Small Cell Lung Cancer, Prostate Cancer. | γ-rays X-rays | [60,62,63,64] |
| Caffeic Acid Phenetyl Ester | 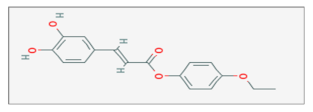 C17H16O5 | Adenocarcinoma, Breast Cancer, Lung Cancer, Medulloblastoma. | γ-rays X-rays | [68,69,70,71,72] |
| Emodin |  C15H10O5 | Cervical Cancer, Hepatocellular Carcinoma, Nasopharyngeal Carcinoma, Sarcoma. | γ-rays X-rays | [79,80,81,82,83] |
| Flavopiridol | 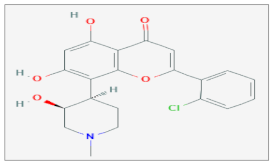 C21H20ClNO5 | Cervix Cancer, Esophageal adenocarcinoma, Esophageal squamous Carcinoma, Glioma, Lung Carcinoma, Ovarian Carcinoma, Prostate Cancer, Zebrafish Model. | γ-rays X-rays | [91,92,93,94,95,101,109,110,111,112]. |
| Berberin | 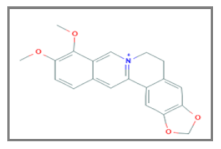 C20H18NO4 | Breast Cancer, Esophageal Carcinoma, Lung Carcinoma, Nasopharyngeal Carcinoma, Osteosarcoma, Prostate Cancer. | γ-rays X-rays | [122,123,124,127,128,134,140] |
| Genistein | 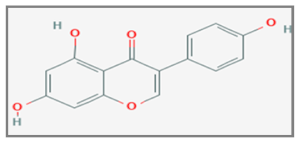 C15H10O5 | Breast Cancer, Cervical Cancer, Non-Small Cell Lung Cancer. | γ-rays X-rays | [148,150,151,152,158,162] |
| Sodium Selenite | 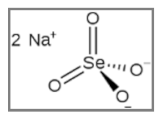 NaSeO3 | Breast Cancer; Glioma; Melanoma. | γ-rays X-rays | [165,166,167] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calvaruso, M.; Pucci, G.; Musso, R.; Bravatà, V.; Cammarata, F.P.; Russo, G.; Forte, G.I.; Minafra, L. Nutraceutical Compounds as Sensitizers for Cancer Treatment in Radiation Therapy. Int. J. Mol. Sci. 2019, 20, 5267. https://doi.org/10.3390/ijms20215267
Calvaruso M, Pucci G, Musso R, Bravatà V, Cammarata FP, Russo G, Forte GI, Minafra L. Nutraceutical Compounds as Sensitizers for Cancer Treatment in Radiation Therapy. International Journal of Molecular Sciences. 2019; 20(21):5267. https://doi.org/10.3390/ijms20215267
Chicago/Turabian StyleCalvaruso, Marco, Gaia Pucci, Rosa Musso, Valentina Bravatà, Francesco P. Cammarata, Giorgio Russo, Giusi I. Forte, and Luigi Minafra. 2019. "Nutraceutical Compounds as Sensitizers for Cancer Treatment in Radiation Therapy" International Journal of Molecular Sciences 20, no. 21: 5267. https://doi.org/10.3390/ijms20215267
APA StyleCalvaruso, M., Pucci, G., Musso, R., Bravatà, V., Cammarata, F. P., Russo, G., Forte, G. I., & Minafra, L. (2019). Nutraceutical Compounds as Sensitizers for Cancer Treatment in Radiation Therapy. International Journal of Molecular Sciences, 20(21), 5267. https://doi.org/10.3390/ijms20215267











